Quantum mechanics is a fundamental theory in physics that describes the behavior of matter and energy at the atomic and subatomic scale. It was developed in the early 20th century as a way to explain the strange and seemingly paradoxical behavior of particles, such as the dual nature of light and the unpredictability of subatomic particles.
Uncertainty Principle
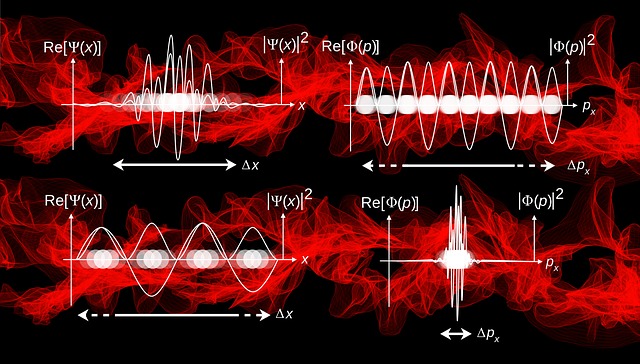
One of the key principles of quantum mechanics is the uncertainty principle, which states that it is impossible to simultaneously know both the exact position and momentum of a subatomic particle with complete accuracy. This principle is a direct result of the wave-particle duality of matter, which means that particles such as electrons can exhibit both wave-like and particle-like properties depending on how they are observed.
Quantization
Quantum mechanics also introduces the idea of quantization, which is the concept that certain physical quantities, such as energy and angular momentum, can only take on certain discrete values rather than being able to take on any value within a certain range. This concept is fundamental to understanding the behavior of atomic and subatomic particles and has led to the development of the quantum theory of the atom.
Superposition
Another important concept in quantum mechanics is the idea of superposition, which states that a particle can exist in multiple states or locations at the same time. This concept has been demonstrated through a number of experiments, such as the double-slit experiment, in which electrons can pass through two slits at the same time and interfere with themselves to produce a wave-like pattern on a screen.
The infamous Schrödinger’s cat
Schrödinger’s cat is a thought experiment, proposed by the physicist Erwin Schrödinger in 1935, that is used to illustrate the strange and counterintuitive nature of quantum mechanics. The thought experiment involves a cat that is placed in a sealed box with a device that has a 50% chance of releasing a poison gas, killing the cat, after a certain amount of time. According to quantum mechanics, until the box is opened and the cat’s fate is observed, the cat is considered to be in a superposition of both alive and dead states.

The thought experiment is meant to illustrate the idea of superposition, which is a fundamental concept in quantum mechanics that states that a particle can exist in multiple states or locations at the same time. It is also meant to illustrate the idea of wave function collapse, which is the process by which the wave function of a quantum system “collapses” into a definite state when it is observed.
Schrödinger’s cat is often used as a way to introduce and explain these concepts to students and laypeople, although it should be noted that the thought experiment is not a real physical situation and is not meant to be taken literally. Instead, it is meant to be a thought experiment that helps to illustrate the counterintuitive and seemingly paradoxical nature of quantum mechanics.
A slight detour: Albert Einstein, however, was never a great fan of quantum mechanics. See this interesting video by National Geography to know his take on the uncertainty principle.
In addition to these fundamental concepts, quantum mechanics also includes a number of mathematical tools and techniques that are used to describe and predict the behavior of quantum systems. These include the Schrödinger equation, which is used to describe the time evolution of a quantum system, and the wave function, which is a mathematical representation of the state of a quantum system.
Applications
One of the key applications of quantum mechanics is in the field of quantum chemistry, which uses the principles of quantum mechanics to understand and predict the behavior of chemical reactions and the properties of molecules. Quantum mechanics has also played a central role in the development of solid-state physics, which is the study of the properties of solids and the ways in which they can be manipulated and controlled.
Quantum mechanics has had a profound impact on our understanding of the nature of reality and has led to the development of many important technologies, including transistors, lasers, and computer memory. It is also an active area of research, with scientists exploring the potential applications of quantum mechanics in fields such as computing, communication, and medicine.
Challenges and Conclusion
One of the key challenges in the field of quantum mechanics is to find ways to reconcile the seemingly strange and counterintuitive predictions of the theory with our classical understanding of the world. Despite this, the theory has been incredibly successful in predicting and explaining the behavior of quantum systems and has been confirmed through a wide range of experiments and observations.
References:
- Feynman, R. P. (1985). QED: The strange theory of light and matter. Princeton University Press.
- Griffiths, D. J. (2005). Introduction to quantum mechanics (2nd ed.). Pearson Prentice Hall.
- Sakurai, J. J. (2011). Modern quantum mechanics (Rev. ed.). Cambridge University Press.
- Weinberg, S. (1992). Dreams of a final theory: The search for the fundamental laws of nature. Vintage Books.
Was this summary helpful?
Mention in the comments section below.
Do not forget to check our other research paper summaries and general blog posts.